|
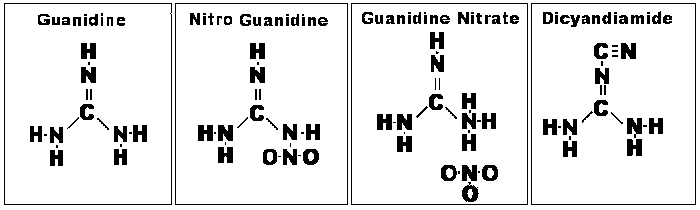
Guanidine
nitrate (GN, NH:C(NH2)2. HNO3).
MP: 215-216ºC
Molecular weight: 122.1
White or creamy grain or
crystal
Applications: raw material for
production of medicines, explosives, coatings, dyes.
Readily soluble in alcohol, very
readily in water, and may be recrystallized from either solvent.
Traditionally, Jetex fuel has used this organic oxidizer, which sometimes comprises more than 90% of the pellet
weight. GN is especially useful as a gas-generating chemical since it is entirely
comprised of gaseous elements and carbon. This permits it to burn without ash, slag,
or residue.
Further, GN propellants decompose
at a rather low temperature. It appears that in decomposition,
nitrous oxide and a small amount of nitrogen are first produced. CO2 comes off later
and more slowly. NH3 is apparently also expelled. The final products of
combustion are probably CO2, water, CO, and nitrogen.
GN is essentially a compound
comprised of guanidine (NH2--C(NH)--NH2 and nitric acid (HNO3) radicals.
Structurally, GN is 2NH2--C(NH)--NH2.HNO3. Each molecule has two molecules of
guanidine combined with one molecule of nitric acid. From this it is apparent that GN
differs chemically from the inorganic nitrates, such as KNO3 and AN.
Guanidine itself is crystalline,
deliquescent, and strongly caustic, taking up CO2 from the air. It is a strong
monoacid base, indistinguishable from KOH in an electrometric titration. It was first obtained by
the oxidation of guanine. This is a substance found in guano and closely related to uric acid; it
is interesting to note that the saltpeter [KNO3 and NaNO3] deposits of history were also
associated with guano deposits, often within caves. In modern use, cyanamide
(NH2--C--N), a widely used organic precursor, can be used to synthesize guanidine.
Cyanamide is produced from coke, limestone, and nitrogen gas.
Guanidine nitrate can be
synthesized from dicyandiamide by the action of aqua regia (a process patented in
1909). More commonly, it is synthesized from dicyandiamide and ammonium nitrate
(AN).
Chemical Structures
|